De novo non-alcoholic fatty liver disease after liver transplantation in small liver transplant centre in Slovakia
Lubomir Skladany1, Svetlana Adamcova Selcanova1, Jana Ciefova1, Natalia Bystrianska1, Beata Skvarkova1, Beata Bachova1, Tomas Koller2.
1HEGITO (Division Hepatology, Gastroenterology and Liver Transplantation), FD Roosevelt Hospital, Banska Bystrica, Slovakia (Slovak Republic); 2Department Internal Medicine, Comenius University Faculty of Medicine , University Hospital Bratislava Ruzinov, Bratislava, Slovakia (Slovak Republic)
Background: Fatty liver disease (NAFLD-non-alcoholic fatty liver disease) is one of the most common causes of-, and indications for- chronic liver diseaseand liver transplant (LTx), respectively. NAFLD arising after LTx for another indication is called the de novo NAFLD.
Aim: To determine by MRI the incidence of de novo NAFLD in patients(pts) after LTx at the Transplant Centre (TC) B. Bystrica (BB), Slovakia.
Patients and Methods: Prospective data analysisof consecutive pts after LTx. Thestudy interval - January 2015 and December 2019. We analysed: 1) anthropometric measurements; 2) Lab Tests needed for FLI (Fatty Liver Index); 3) MR spectroscopy (MRS) and MR Elastography (MRE).
Inclusion criteria: LTx in TC BB, for cirrhosis of non-NAFLD etiology.
Exclusion criteria:LTx for NAFLD, early death (<3m), severe complications after LTx (>2m).
Recorded variables: Etiology,sex, age, Child-Pugh, MELD(Model for End Stage Liver Disease), BMI (Body mass index) at months 3, 6, 12, 24 after LTx,waist circumference, GGT(gamaglutamyltransferase), TAG (triacylglyceride), FLI at the same intervals after LTx (NAFLD≥60 = NAFLD [www.medal.org)] , MRS and MRE at months 6, 12, and 24 (MRS ≥5% = NAFLD; MRE ≥2,88kPa = signifficant fibrosis).
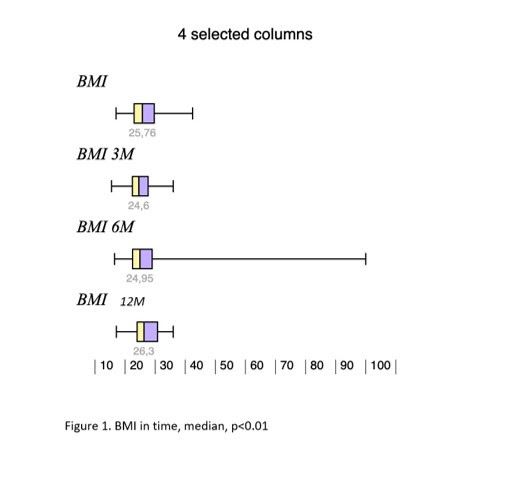
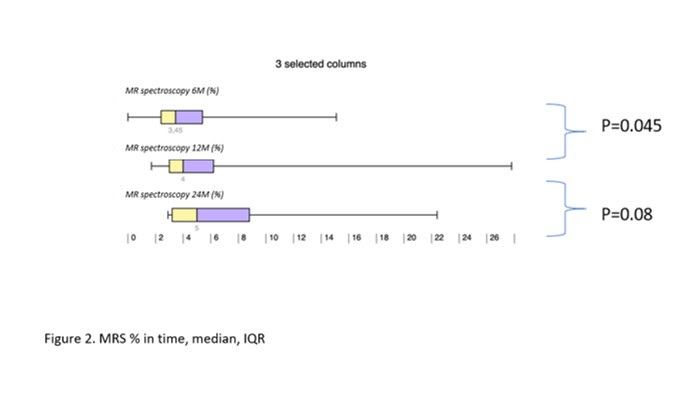
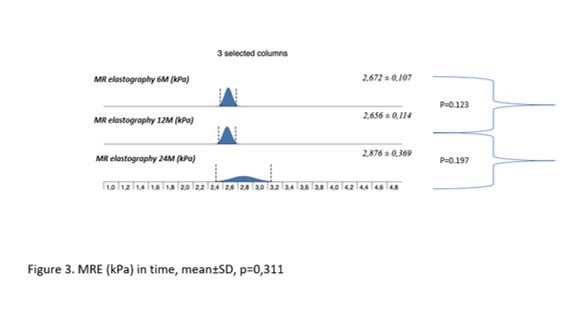
Results: During the study interval, 164 LTx were performed in 153pts.We excluded 6 pts(4%) transplanted for NAFLD and 52(34%) for pre-defined criteria.We included 95pts(62%), aged 50,4 years(18-70), 38% women, with MELD15,7p(7-34).Etiology of ACLD: ALD 44pts(46%), PSC 16(17%),AIH 12(13%), miscellaneous 8(9%), HCC 7(7%), viral hepatitis 3(3%), Secondary biliary cholangitis (SBC) 3 (3%), Wilson‘sdisease 2(2%).BMI:baseline - 25,76; 3m-24,6; 6m-24,95; 12m-26,5 (p < 0,01).FLI:3m-46.00 (24.00, 72.00); 6m-53.00 (30.50, 77.00); 12m-51.00 (22.00, 79.00); (p=NS).MRS:6m-3,45,12m – 4(p=0,045); 24m – 5(p=0,08). MRS>5%: 6m-26,5%; 12m-32,1%; 24m-42,9%;(p=0,368).MRE (mean ±SD, p=0,368): 6m - 2,672 ±0,107kPa, 12m - 2,656±0,114 (p=0,123); 24m - 2,876±0,369; (p=0,197).MRE≥2,88kPa: 6m-26,8%, 2m-29,6%; (p=0,07); 24m-46,2%; (p=0,1).
Conclusion: We identified risingliver fat content and BMI over time after LTx. Six months after LTx, MRS detected liver fat>5% in 1/4 of pts. Six, and 12 m after LTx, fibrosis was present in 26%, and 29% of pts, respectively. Clinical impact is not known.
There are no comments yet...