Hepatitis B immunoglobulin prophylaxis for prevention of de novo hepatitis B infection from hepatitis B core antibody-positive donors
Kyeongdeok Kim1, Manuel Lim1, Jieun Kwon1, Eunsung Jeong1, Jaehun Yang1, Okjoo Lee1, Sang Jin Kim1, Jinsoo Rhu1, Kyo Won Lee1, Jae Berm Park1, Jong Man Kim1, Gyu Seong Choi1, Jae-Won Joh1.
1Department of Surgery, Samsung Medical Center, Seoul, Korea
Introduction: Recently, hepatitis B core antibody (anti-HBc) positive liver grafts are used as extended criteria donor organs. However, prophylaxis methods to prevent de novo hepatitis B virus (HBV) infection after liver transplantation (LT) are still controversial. Thus, the purpose of our study is to evaluate the risk and outcomes of receiving anti-HBc positive grafts with prophylactic HBIG monotherapy.
Method: All adult patients who underwent LT at Samsung Medical Center in Seoul, Korea, between January 2001 and December 2018 were gathered from a prospectively maintained database and reviewed retrospectively. HBV patients received the combination treatments with antiviral prophylaxis and hepatitis B immunoglobulin (HBIG). Prophylactic HBIG monotherapy was given for patients without HBV receiving grafts from anti-HBc positive donors.
Results: A total of 1355 patients underwent LT during this period. Among them, 457 (33.7%) were anti-HBc positive and 898 (66.3%) were anti-HBc negative donors, who underwent follow-up for a median time of 5.7 years. Perioperative outcomes (mortality in 30 days, complications and postoperative ICU stay period) were similar between the two groups. Also, there were no significant difference in the 1-, 5-, 10- and 15-year patient survival rates between the anti-HBc positive (87.5%, 73.5%, 67.7% and 61.2%) and the anti-HBc negative groups (88.5%, 77.7%, 70.7% and 69.6%, p = 0.080). All of the 117 recipients who were HBsAg negative and received anti-HBc positive grafts were given prophylactic HBIG monotherapy. Only one patient (0.9%, 1/117) developed de novo HBV during follow-up. There were 972 HBsAg-positive patients and 348 (35.8%) received anti-HBc positive grafts. The risk of HBV recurrence was similar between 2 groups (p = 0.308). The donor anti-HBc status did not have a significant influence on the long-term survival or the risk of de novo or recurrent HBV infection after LT.
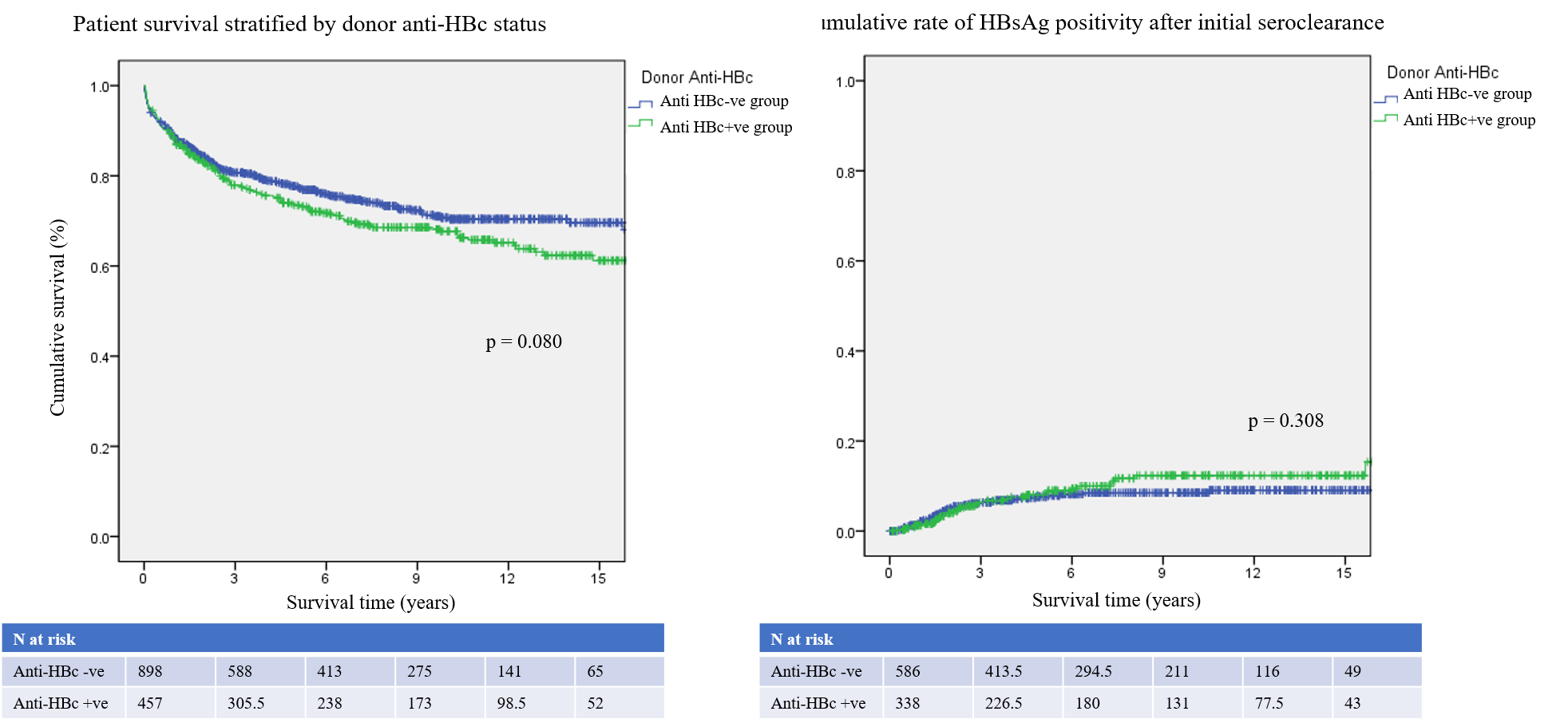
Conclusion: De novo HBV was very rare especially with HBIG prophylaxis in non-HBV patients received anti-HBc positive liver graft. There was no significant influence of anti-HBc positive grafts on the perioperative and long-term outcomes after LT.
There are no comments yet...