Global, multicentre, non-interventional study to assess long-term outcomes in kidney transplant recipients converting from twice-daily, immediate-release tacrolimus to once-daily, prolonged-release tacrolimus: 4-year interim analysis of the CHORUS study
Nassim Kamar1, László Kóbori2, Swapneel Anaokar3, Martin Blogg3.
1Department of Nephrology and Organ Transplantation, CHU Rangueil, Toulouse, France; 2Department of Transplantation and Surgery, Semmelweis University, Budapest, Hungary; 3Astellas Pharma Europe, Ltd, Addlestone, United Kingdom
The CHORUS Study.
Introduction: Potential benefits of converting from twice-daily, immediate-release tacrolimus (IRT) formulations to once-daily, prolonged-release tacrolimus (PRT) include improved medication adherence, decreased variability in tacrolimus exposure and more stable renal function. This study was undertaken to assess long-term outcomes in kidney transplant recipients (KTRs) over 5 years following conversion from IRT to PRT under routine practice conditions. We present an interim analysis with 4 years of follow up.
Materials and Methods: This was a multicentre, non-interventional study (NCT02555787) in adult KTRs converting from IRT to PRT (Advagraf®, Astellas Pharma Europe, Ltd). The primary endpoint was change in renal function (eGFR, calculated by Modified Diet in Renal Disease 4). Secondary assessments included tacrolimus dose and trough levels, Kaplan–Meier estimated rates of biopsy-confirmed acute rejection (BCAR)-free, graft and patient survival, emergence of donor-specific antibodies (DSA) and safety. Patients were grouped by time of conversion post-transplant (early, ≤6 months; late, >6 months).
Results: Overall, 4372 KTRs were enrolled; 4104 formed the full analysis set (early converters, 1092; late converters, 3012). Baseline characteristics were similar between groups; overall, mean (standard deviation) age was 51.0 (13.3) years. Mean time from transplantation to conversion was 2.3 (1.7) months for early converters vs 64.6 (59.4) months for late converters. Mean PRT dose was 7.02 (4.08) vs 3.91 (2.47) mg/day at conversion and 3.94 (2.18) vs 3.90 (2.29) mg/day at 48 months after conversion, in early vs late converters, respectively (Table 1).
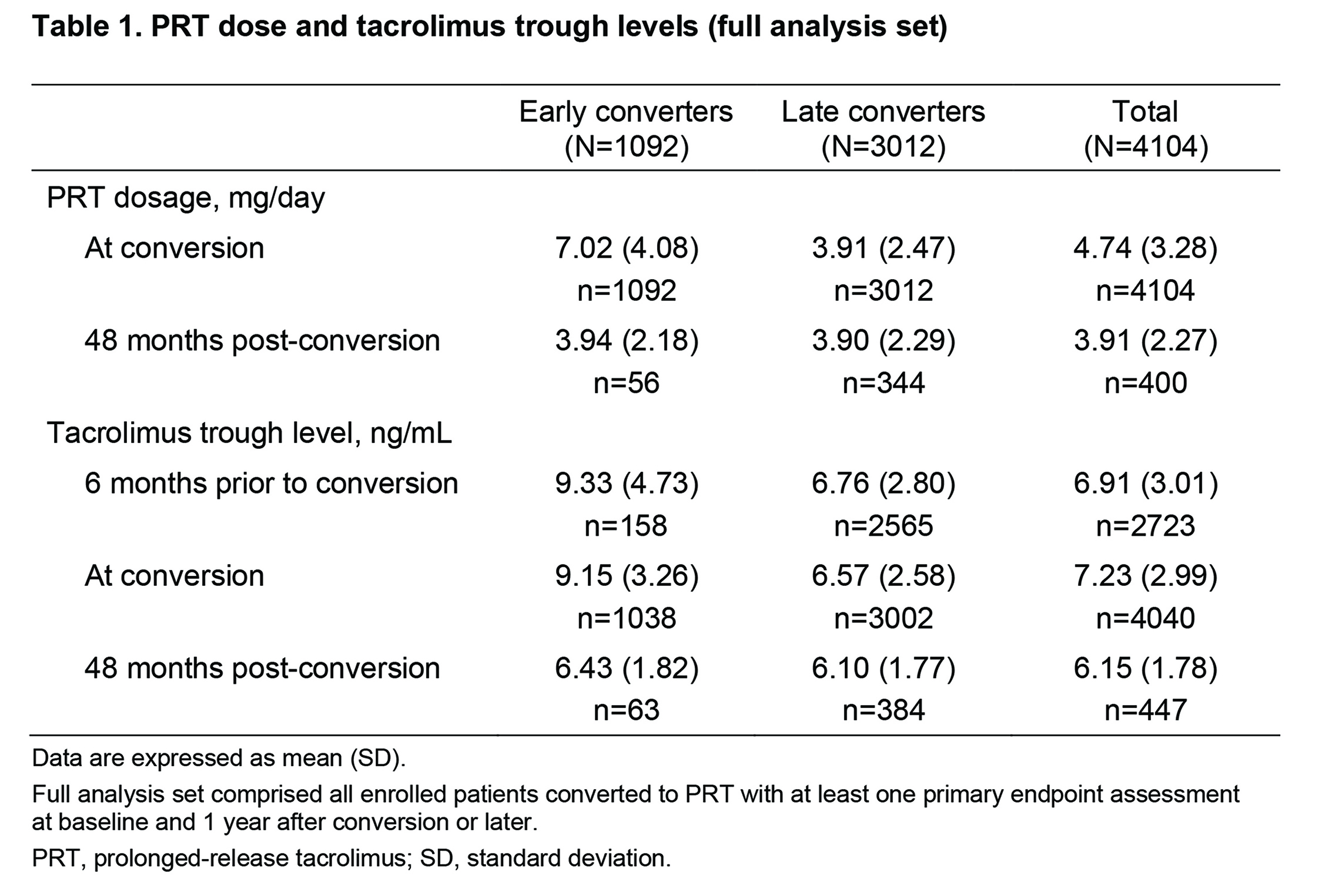
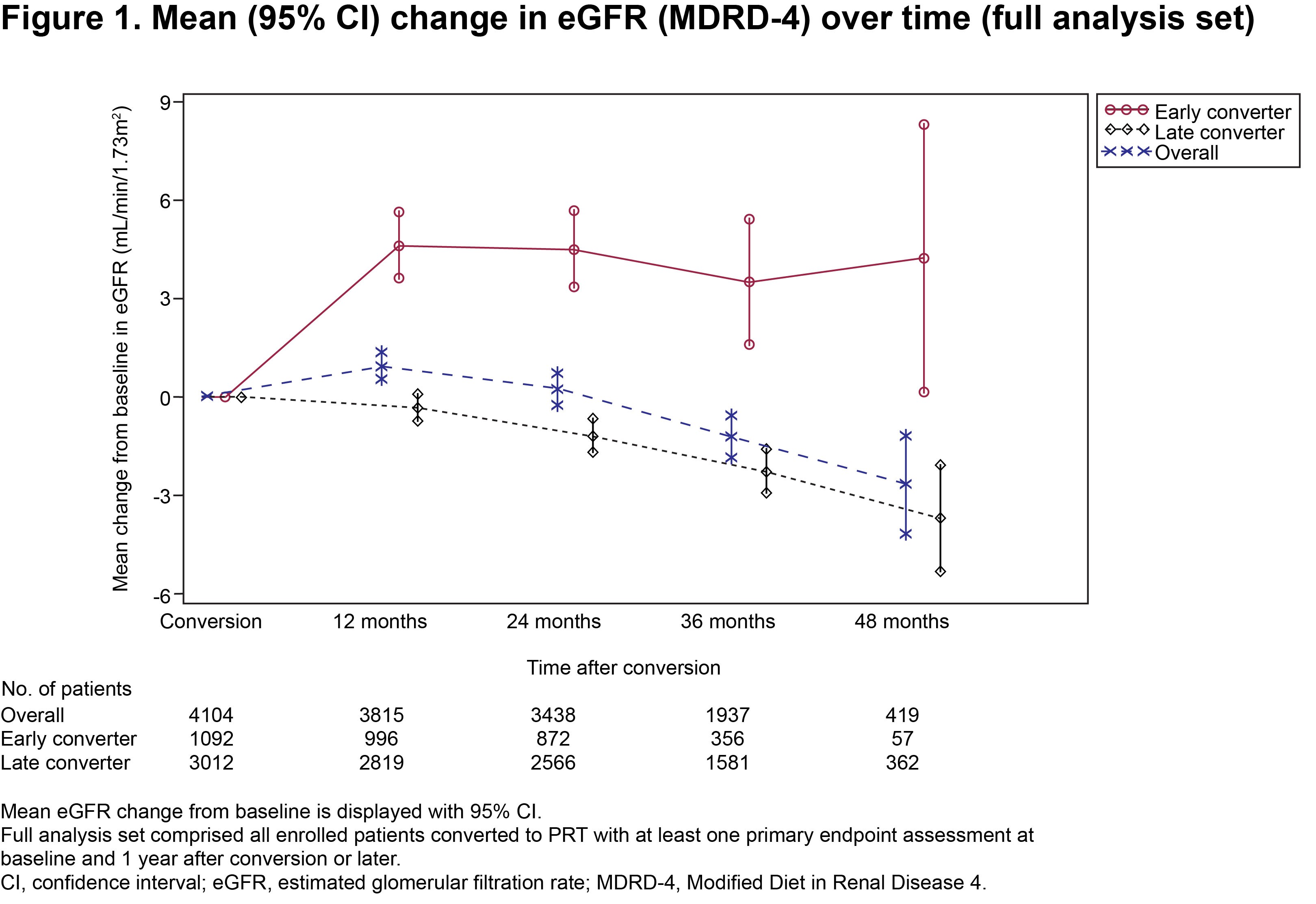
Respective mean duration of PRT treatment was 776.0 (323.6) vs 915.0 (335.1) days. Mean tacrolimus trough levels were 9.15 (3.26) vs 6.57 (2.58) ng/mL at conversion and 6.43 (1.82) vs 6.10 (1.77) ng/mL at 48 months, in the two groups, respectively. Mean change in eGFR from time of conversion to 48 months post-conversion was +4.26 (15.39) mL/min/1.73m2 in early converters vs −3.66 (15.34) mL/min/1.73m2 in late converters (Fig. 1).
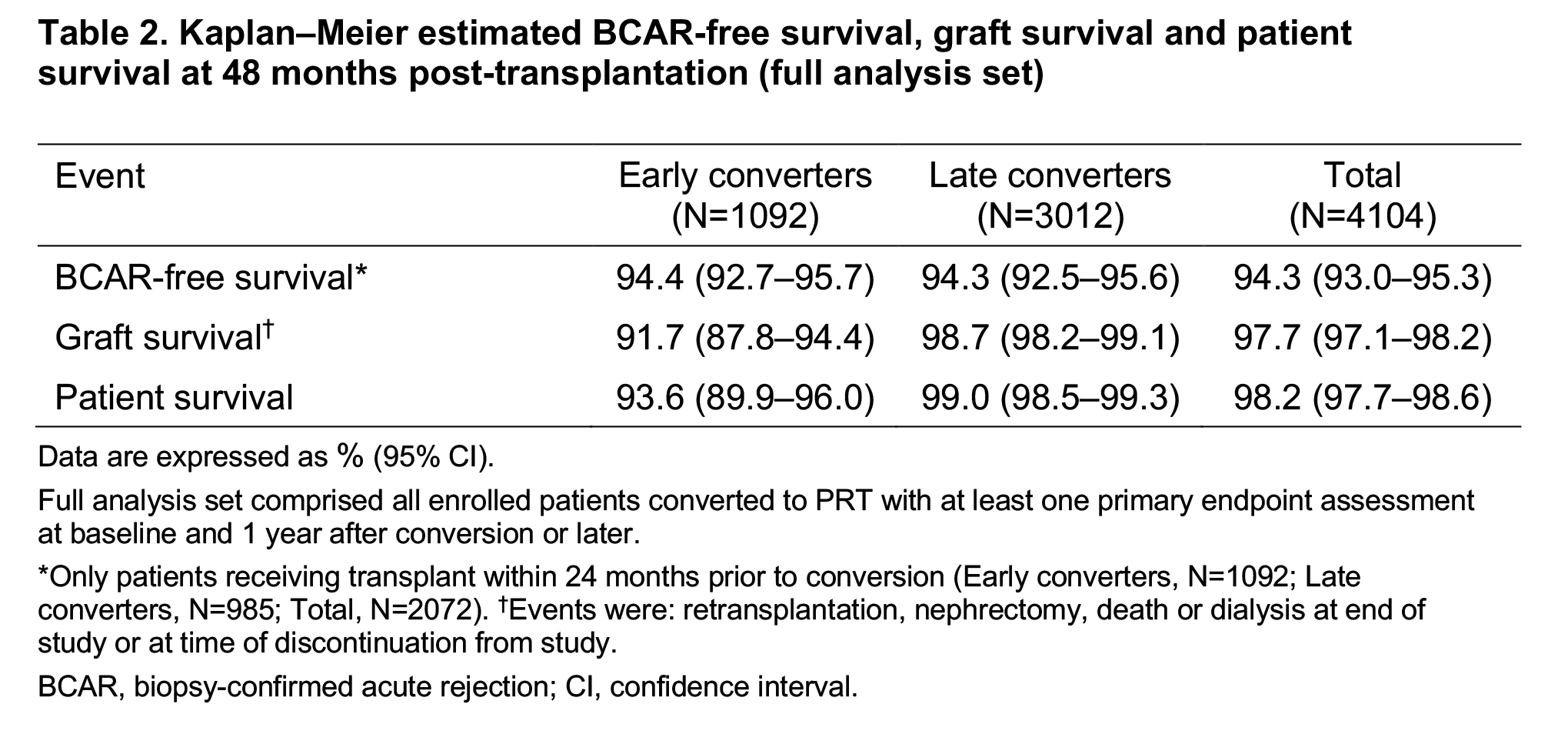
Kaplan–Meier estimated BCAR-free survival, graft survival and patient survival rates were similar between the two groups at 48 months post-transplant (Table 2).
Among patients with data (the majority had missing DSA data), the proportion with DSA before or at conversion was 15.2% in early converters vs 19.7% in late converters; proportions with DSA on at least one post-conversion assessment were 25.7% vs 24.4%. Adverse events (enrolled-patient set) were reported by 67.9% of early (n=1252) vs 55.9% of late (n=3120) converters (considered treatment-related in 20.0% vs 12.5%); incidences of treatment-related serious adverse events were 9.9% vs 5.6%.
Conclusion: Consistent outcomes were observed in this interim analysis of a large population of KTRs at 4 years of follow up after conversion from IRT to PRT. Relevant clinical differences will be assessed at the end of the study.
Astellas Pharma Europe Ltd.
There are no comments yet...