
Dr. Kin Pan Au graduated from the medical school of the University of Hong Kong in 2009 and completed General Surgery residency in Queen Mary Hospital. Upon completion of his fellowship training in 2016, he went on to subspecialize in hepatobiliary and pancreatic surgery, and liver transplantation.
Besides concentrating on the clinical training, he demonstrated enthusiasm and endurance in surgical research. He was awarded second prize of the best research award of the year by the College of Surgeons of Hong Kong in 2014 and best research output by the Department of Surgery, the University of Hong Kong in 2015. He was also the awardee of various travel grants and awards from different international conferences over the years. His publications emphasized on liver volumes, graft functions and rejections in field of liver transplantation.
Outside of clinical services, he has been appointed as the deputy chief of residents in 2017 and the chief of residents in 2018. He actively participated in medical education of medical students and young residents and had organized a variety of training workshops in both hospital and clinical laboratory.
Outside of medicine, he enjoys hiking and has been a trailwalker. He loves travelling and exploring cultures and landscapes of different countries.
Mammalian target of rapamycin Inhibitors after post-transplant hepatocellular carcinoma recurrence: Is it too late?
Kin Pan Au1, Kenneth Siu Ho Chok1.
1Surgery, The University of Hong Kong, Hong Kong, Hong Kong
Background: Mammalian target of rapamycin (mTOR) inhibitors have been shown to reduce the risk of tumour recurrence after liver transplantation for hepatocellular carcinoma (HCC). However, its role in established post-transplant HCC recurrence is uncertain. We investigate whether mTOR inhibitor offers survival benefit in this context.
Methods: A retrospective study of 143 patients who developed HCC recurrence after liver transplantation was performed. They were divided into 2 groups based on whether they had received mTOR inhibitor-based immunosuppression. The primary endpoint was post-recurrence survival.
Results: Seventy-nine (55%) patients received mTOR inhibitor-based immunosuppressive regime while 64 (45%) of them did not. The mTOR inhibitor group had a lower number of recurrent tumours (2 vs. 5, p=0.02) and received more active treatments including radiotherapy (39 vs. 22%, p=0.03) and targeted therapy (59 vs. 23%, p<0.001). The median post-recurrence survival was 21.0±4.1 months in mTOR inhibitor group and 11.2±2.5 months in the control group. Multivariate cox regression analysis confirmed mTOR inhibitor was independently associated with improved post-recurrence survival (p=0.04, OR 0.482, 95%CI 0.241-0.966).
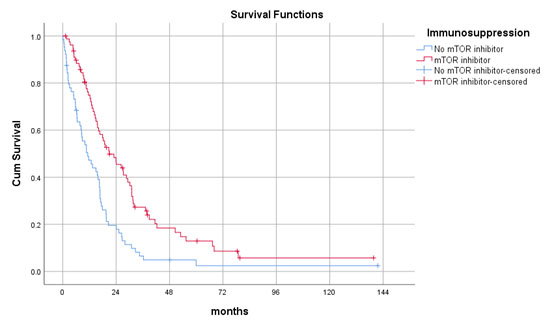
Number of recurrent tumours and use of other treatment modalities did not affect survivals. There were no survival differences observed between mTOR inhibitor monotherapy and combination therapy with calcineurin inhibitor (CNI).
Conclusions: mTOR inhibitors prolonged survival after post-transplant HCC recurrence.
[1] Rodríguez-PerálvarezM, TsochatzisE, NaveasMC, et al. Reduced exposure to calcineurin inhibitors early after liver transplantation prevents recurrence of hepatocellular carcinoma. J Hepatol. 2013;59(6):1193-1199.
[2] TosoC, MeraniS, BigamDL, ShapiroAMJ, KnetemanNM. Sirolimus-based immunosuppression is associated with increased survival after liver transplantation for hepatocellular carcinoma. Hepatology. 2010;51(4):1237-1243.
[3] AuKP, ChokKSH. Multidisciplinary approach for post-liver transplant recurrence of hepatocellular carcinoma: A proposed management algorithm. World J Gastroenterol. 2018;24(45):5081-5094.
[4] GeisslerEK, SchnitzbauerAA, ZülkeC, et al. Sirolimus Use in Liver Transplant Recipients With Hepatocellular Carcinoma: A Randomized, Multicenter, Open-Label Phase 3 Trial. Transplantation. 2016;100(1):116-125.
There are no comments yet...