Determination of type 2 diabetes mellitus risk in potential living kidney donors: A single center protocol and experience
Golnaz Friedman1,3, Olivia Moss1,3, Muna Alnimri1, Kalyani Chandra1, Anthony El-Sokkary1, John Yoon2, Yihung Huang1, Ling-Xin Chen1.
1Transplant Nephrology, University of California, Davis, Sacramento, CA, United States; 2Endocrinology, University of California, Davis, Sacramento, CA, United States; 3Food and Nutrition Services, University of California, Davis, Sacramento, CA, United States
Introduction: Top reasons potential living donors (PLDs) are denied for donation are lifestyle-related conditions such as obesity, HTN, and pre-diabetes1. In recent years, the rates of obese donors with glucose intolerance has increased2. While type 2 diabetes mellitus (DM2) is an absolute contraindication for donation, proper identification of future development of DM2 in PLDs remains a challenge. It is possible for PLDs with normal metabolic labs or BMI to have high DM2 risk due to lifestyle factors, so nutrition evaluation for all PLDs is vital. Here, we present a formalized protocol for DM2 risk assessment in PLDs.
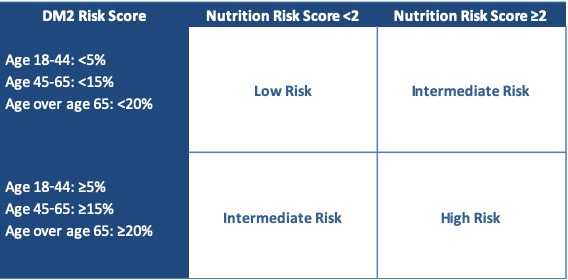
Materials & Methods: An evaluation by a Registered Dietitian (RD) was developed to standardize nutrition assessments of all PLDs. This assessment included: weight, family history of DM, ethnicity, labs, physical activity, and diet, resulting in a risk score of 0-3. Objective assessment of DM2 risk was also made using the Atherosclerosis in the Community (ARIC) 2016 calculator to estimate 10-year DM2 risk for each PLD3. CDC data on DM2 risk in the general population was used to develop acceptable age-based cutoffs for DM2 risk. Based on the RD evaluation and the ARIC 2016 risk score, PLDs were placed into a high risk (HR), intermediate risk (IR), or low risk (LR) category (Table 1). For PLDs in the IR category, fasting serum insulin was obtained to identify early insulin resistance4. Here, we present data from PLDs evaluated between 1/1/17-12/31/18. Charts were reviewed for donation case outcomes. Chi-squared and ANOVA tests were used to compare characteristics between groups.
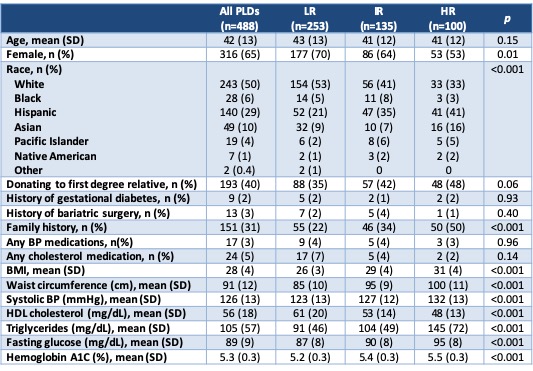
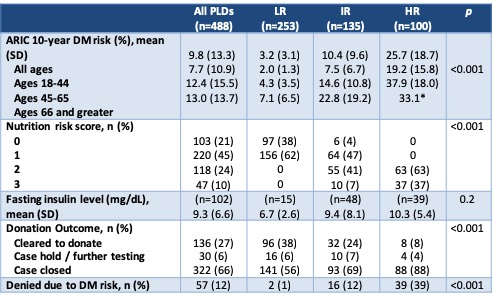
Results and Discussion: A total of 488 PLDs were evaluated in the 2-year period. As expected, variables that are part of the ARIC 2016 risk calculator differed significantly between DM2 risk groups (Table 2). Donation to a first degree relative also trended toward significance, which is expected due to prevalence of DM2 in the recipient population. Overall, 27% of PLDs were cleared for donation after full evaluation (Table 3). In the LR group (n=253), 2 were still denied for DM2 risk based on clinical judgement. In the HR group (n=100), 8 were cleared to donate because they made appropriate lifestyle changes after initial evaluation and 4 were placed on hold for lifestyle modification at the time of data collection. In the IR group, 24% were cleared to donate and 69% were denied, of which 16 were denied due to DM2 risk.
Conclusion: This protocol has allowed our team to increase the number of PLDs approved for donation that may have been screened out based on the traditional practice of using BMI or abnormal glycemic labs alone. We have increased living donation through a more all-encompassing assessment of DM2 risk based on lifestyle factors and objective clinical indicators. Furthermore, we have been able to better counsel our PLDs on their actual DM2 risk and identify personalized areas for risk mitigation.
[1] Pieloch D, et. al. Progress in Transplantation. 2017; 27: 281-285.
[2] Taler SJ, et. al. Am J Transplant. 2013; 13: 390-8.
[3] Lacy ME, et. al. Diabetes Care. 2016; 39: 285-91.
[4] Johnson JL, et. al. Endocr Pract. 2010; 16: 47-52.