Empagliflozin, a sodium-glucose cotransporter 2 inhibitor, improves donor heart recovery after prolonged cold storage in an isolated working rat heart model
Jeanette E. Villanueva1, Ling Gao1, Aoife Doyle1, Sarah E. Scheuer1, Mark Hicks1,2, Peter S. Macdonald1,3.
1Cardiac Physiology and Transplantation, Victor Chang Cardiac Research Institute, Sydney, Australia; 2Department of Clinical Pharmacology & Toxicology, St Vincent's Hospital, Sydney, Australia; 3Heart and Lung Transplant Unit, St Vincent's Hospital, Sydney, Australia
Introduction: Many donor hearts are unsuitable for transplantation due to ischemia-reperfusion injury during retrieval. Improved donor heart recovery is observed after preservation solution supplementation with sodium-hydrogen exchange inhibitors (NHEI) cariporide/zoniporide however these NHEI are no longer approved for clinical use due to neurotoxicity observed in cariporide clinical trials. One promising alternative is the sodium-glucose cotransporter 2 inhibitor Empagliflozin (Empa) – the first anti-diabetic drug to yield positive cardiovascular outcomes, reduce heart failure hospitalisations and mortality. In vitro studies have shown Empa mimics NHEI and slows pH recovery in cardiomyocytes following an acidic load. Whether Empa is beneficial in the context of donor heart preservation is unknown.
Aim: To test Empa-supplementation during prolonged cold storage of donor hearts.
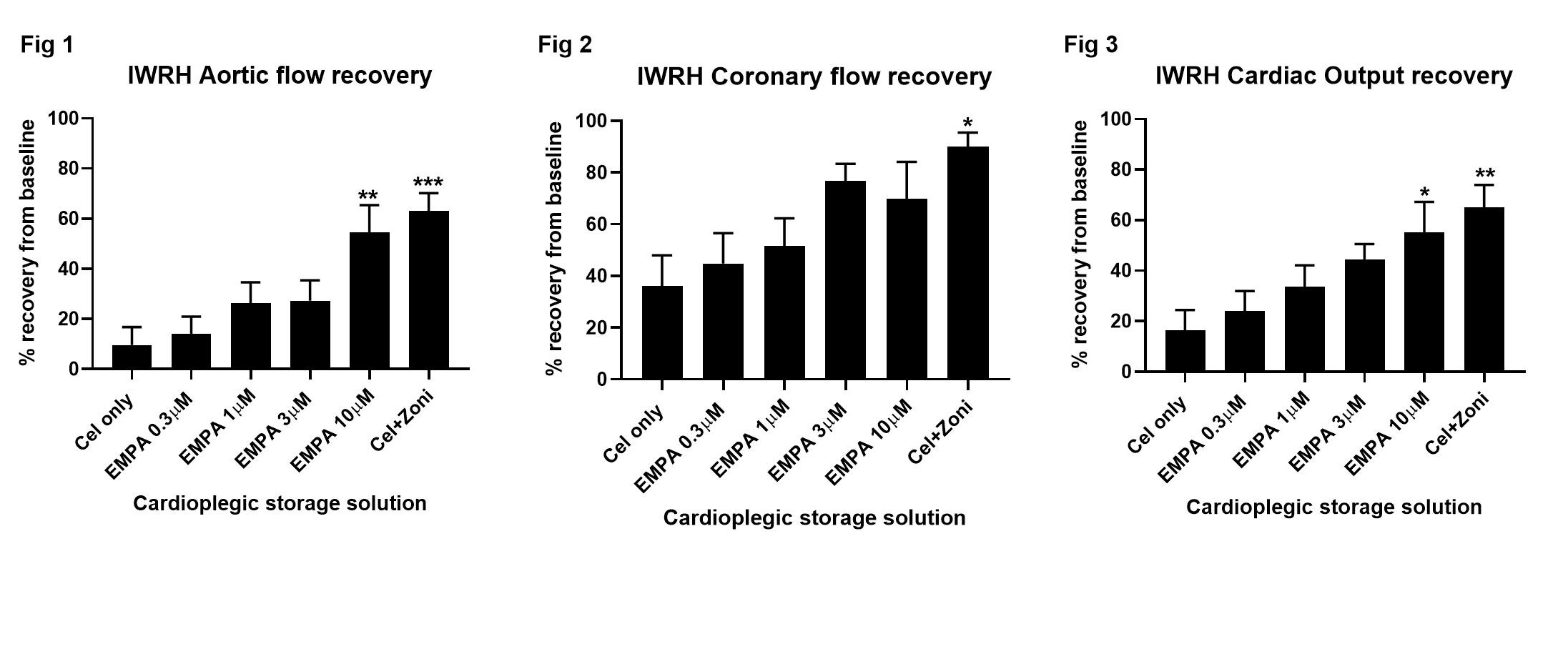
Materials and Methods:: Wistar rat (320–420g; n=5-7) hearts were perfused ex vivo with Krebs-Henseleit buffer (KHB) and baseline hemodynamic measurements acquired. Hearts were arrested and stored in Celsior±0.3–10µM Empa, or Celsior+zoniporide (1µM) (6h, 4°C) then reperfused (KHB, 37°C, Langendorff 15min, working 30min). Post-reperfusion aortic flow (AF), coronary flow (CF), and cardiac output (CO=AF+CF) were expressed as a percentage of baseline measurements. Coronary effluent (pre-storage, 15, 30, and 45 min reperfusion) was assessed for lactate dehydrogenase (LDH) release. Statistical significance was determined using one-way ANOVA (functional studies), or two-way ANOVA (LDH studies) with Tukey’s multiple comparisons test.
Results and Discussion: Donor hearts supplemented with Empa during cold storage showed a dose-dependent improvement in cardiac functional recovery. Compared to unsupplemented control hearts, 10µM Empa-supplementation significantly improved AF (54±11% vs 10±7%, p=0.006; Fig 1), showed a trend for increased CF (70±14% vs 36±12%, Fig 2), and significantly improved CO (55±12 vs 16±8, p=0.03; Fig 3) recovery. The improved functional hemodynamics observed after 10µM Empa-supplementation were comparable to hearts supplemented with 1µM zoniporide which showed significantly improved AF (63±7%, p=0.002 vs control, Fig 1), CF (90±6%, p=0.039, Fig 2) and CO (65±9%, p=0.01, Fig 3) recovery. A trend for reduced LDH efflux, a marker of cellular injury, was observed at 30 min reperfusion in hearts supplemented with 1-10µM Empa or 1µM zoniporide when compared to control hearts.
Conclusion: Supplementation of the cardiac preservation solution with 10µM Empa yields significantly improved functional recovery after prolonged cold storage and reduced cellular injury, with similar cardioprotective efficacy to the NHEI zoniporide. The safety profile and approved clinical use of Empa highlights its potential as a suitable alternative for traditional NHEI (e.g., zoniporide and cariporide) in donor heart preservation.
St Vincent's Clinic Foundation. National Health and Medical Research Council.