Pneumocystis pneumonia occurrence and prophylaxis duration in kidney transplant recipients according to perioperative treatment with rituximab
Hyunwook Kwon1, Dong Hyun Kim1, Youngmin Ko1, Sung Shin1, Young Hoon Kim1, Duck Jong Han1.
1Division of Kidney and Pancreas Transplantation, Department of Surgery, Asan Medical Center, Seoul, Korea
Background: Pneumocystis pneumonia (PCP) is a life-threatening fungal infection that can occur in kidney transplantation (KT) recipients. A growing number of KT recipients are receiving perioperative treatment with rituximab, which is associated with prolonged B-cell depletion and possible risk of PCP occurrence; however, the optimal prophylaxis duration according to rituximab treatment is yet unknown. We compared the occurrence of PCP and the duration of prophylaxis in KT recipients according to rituximab treatment.
Methods: We retrospectively analyzed 2110 patients who underwent KT between January 2009 and December 2016, who were divided into non-Rituximab group (n = 1588, 75.3%) and rituximab group (n = 522, 24.7%).
Results: In the rituximab group, the estimated number needed to treat (NNT) for prophylaxis prolongation from 6 to 12 months was 29.0 with a relative risk reduction of 90.0%. In the non-rituximab group, the estimated NNT value was 133.3 and the relative risk reduction was 66.4%. Rituximab treatment (hazard ratio (HR) = 3.09; P < 0.01) and acute rejection (HR = 2.19; P = 0.03) were significant risk factors for PCP in multivariate analysis.
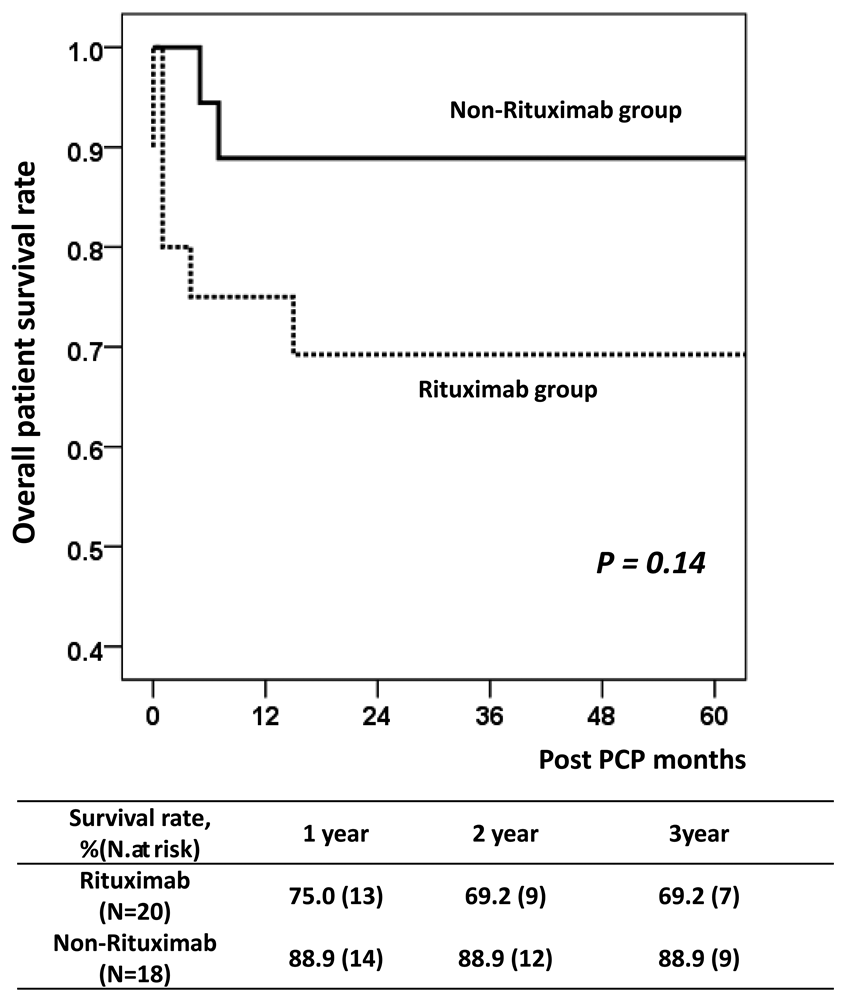
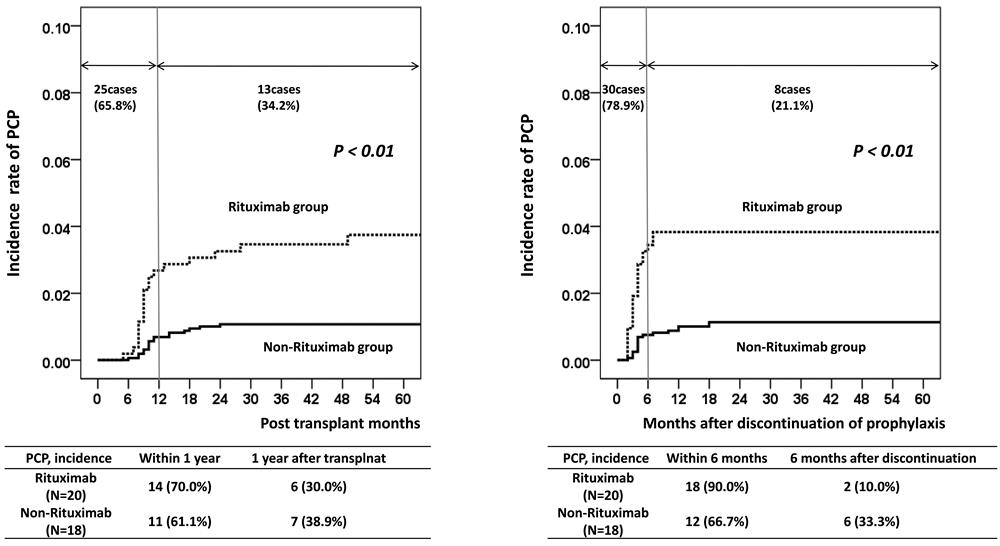
Conclusion: Our results suggest that maintaining PCP prophylaxis for 12 months may be beneficial in KT recipients treated with rituximab for desensitization or acute rejection treatment.