Identification of a novel subset of xeno-antigen specific memory CD4+Foxp3+Tregs in islet-xenotransplant tolerance
Leigh Nicholson1, Yuanfei Zhao1, Yi Wen Qian1, SooLing Tang1, Yi Vee Chew1, Heather Burns1, Geoff Zhang2, Shounan Yi1, Natasha Rogers1, Wayne Hawthorne1, Steve Alexander2, Phillip O'Connell1, Min Hu1.
1Centre for Transplant and Renal Research, Westmead Institute for Medical Research, Westmead, Australia; 2Centre for Kidney Research, The Children's Hospital at Westmead, Westmead, Australia
Background: Blockade of the B7-CD28 and CD40-CD154 pathways induces tolerance towards porcine-islet-cell-cluster (NICC) xenografts in mice.
Aims: 1) Investigate CD4+Foxp3+Tregs in mouse-model of NICC xenograft-tolerance, 2) identify in situ immune-cell subtypes using imaging mass cytometry (IMC) and 3) assess the function of these Foxp3+Tregs.
Methods: C57BL/6-DEREG-mice with diphtheria-toxin(DT)-receptor/GFP attached to Foxp3-gene were transplanted with NICC under renal-capsule. Recipient-mice received CTLA-4-Fc and MR-1 mAb. Foxp3+Tregs were depleted by DT from day 3-17 (induction-phase), or 80-100 days post-transplantation (maintenance-phase). NICC-xenograft function determined by insulin/glucagon staining and serum porcine-c-peptide concentration. A 28-antibody panel for IMC was used to identify in situ cells and a bioinformatics pipeline was created for data analysis.
Results: More CD4+Foxp3+Tregs were seen in NICC-xenografts of treated mice on day-8 and 100 than day-20. Foxp3+ cells increased significantly in the draining-lymph-nodes of treated mice on day-8 (P=0.0010) and 100 (P=0.0128) when compared to the untreated/rejecting groups. CD4+Foxp3+Treg depletion during induction- or maintenance-phase led to rapid xenograft rejection. CD4+Foxp3+Tregs from lymphoid-organs of graft-tolerant DEREG-mice showed a significant increase in CD127, downregulated CD25, and reduced CD27 and CD62L expression compared to naïve/rejecting xenografts. IMC successfully quantified in situ immune-cell subsets (Fig.1), showing increased CD4+CD25-Foxp3+Tregs in tolerant compared to rejecting grafts, and localized within grafts at day-20. CD127+hiCD25+/lowCD44+hiCD62L-CD27-CD4+Foxp3+Tregs were transferred to NICC-transplanted Rag-\- mice and had a greater suppressive capacity than naïve-Tregs.
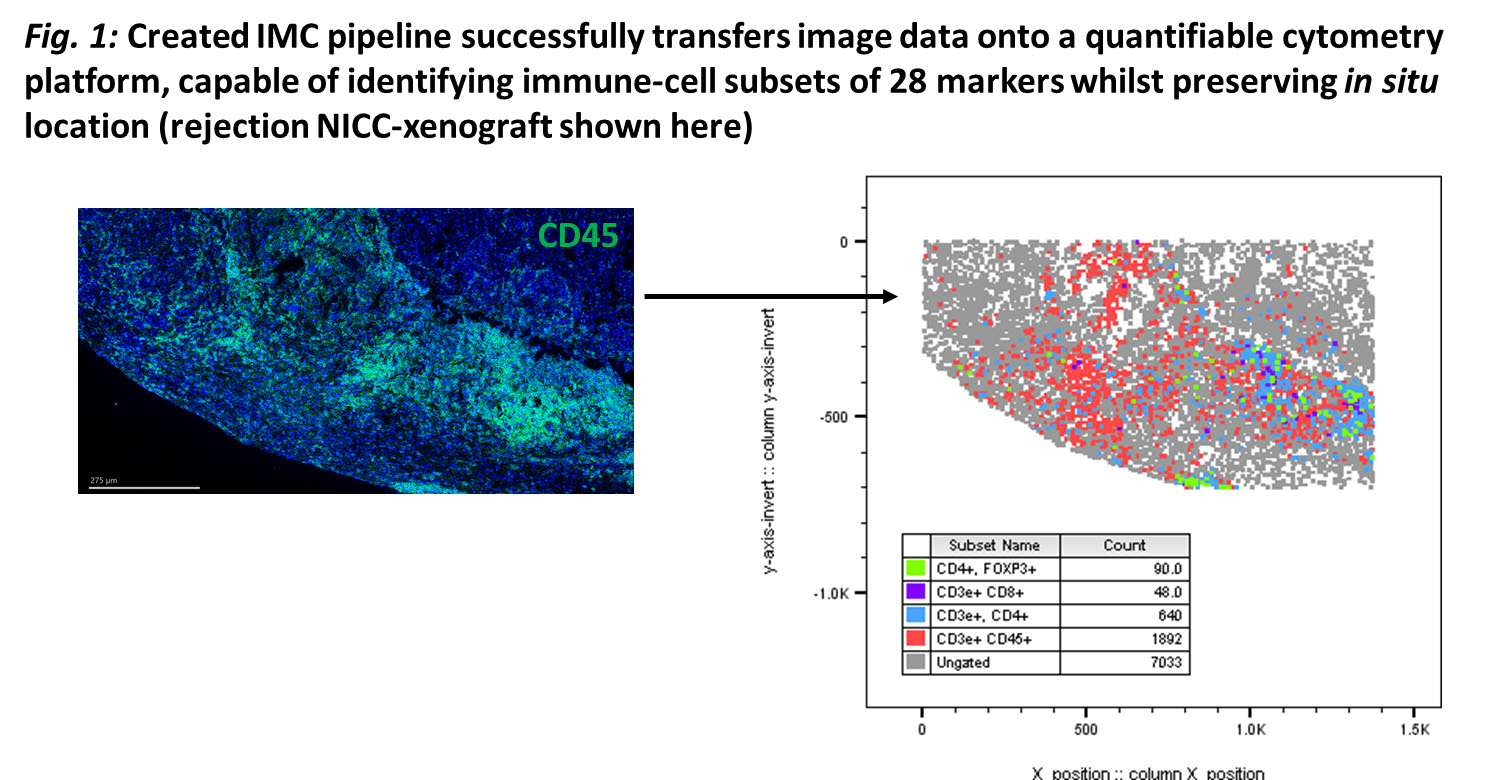
Conclusion: Foxp3+Tregs are essential for NICC-xenograft survival in this model. Importantly, CD127+hiCD44+hiCD62L-CD27-CD25+/lowCD4+Foxp3+Tregs were a subpopulation of Tregs that showed features of antigen-specific memory.
National Health and Medical Research Council of Australia (NHMRC; APP1125456). Juvenile Diabetes Research Foundation/Australian Research Council (JDRF; APP10373210).
There are no comments yet...