Extracellular vesicles from urine as biomarkers of kidney allograft injury: optimization of extracellular vesicle isolation and characterization
Ivana Sedej1, Magda Tušek Žnidarič2, Vita Dolžan3, Miha Arnol1, Metka Lenassi3.
1Department of Nephrology, University Medical Centre Ljubljana, Ljubljana, Slovenia; 2National Institute of Biology, Ljubljana, Slovenia; 3Institute of Biochemistry, Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia
Introduction: In transplant medicine, there is a great need for individualized strategies in terms of early recognition, prevention of allograft injury and immunosuppression therapy adjustment to improve allograft and patient outcomes. Kidney biopsy, a gold standard for the assessment of allograft injury cannot be used as a screening method for the diagnosis of subclinical injury due to it’s invasiveness and possible sampling errors. Contrarily, urine is easily accessible, conveying important and direct information in a form of non-invasive biomarkers regarding kidney allograft injury. The principal, stable carriers of pathophysiological signals with great biomarker potential are extracellular vesicles (EVs). Hence, we focused on characterization of EVs from urine of kidney transplant recipients. Our aim was to establish the method for isolation of urinary EVs that would enable consistent and reliable identification of their characteristics and cargo.
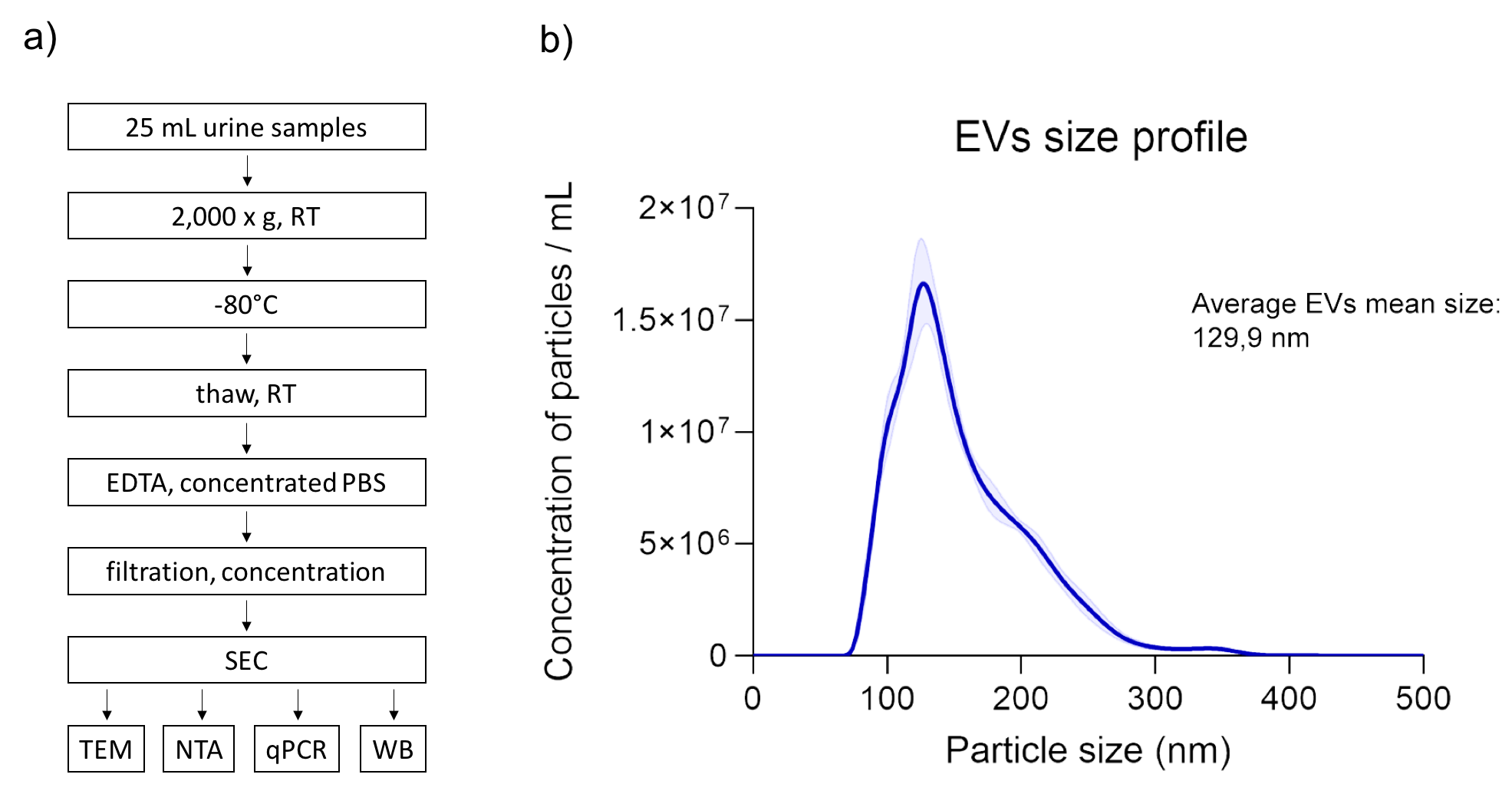
Materials and Methods: The second morning spot urine (25 mL) was collected from 7 kidney allograft recipients and processed within 4 hours. Oxalate precipitation, pH and dilution variability, uromodulin polymerization and high protein concentration were considered when optimizing the method for EV isolation. Morphological characteristics and purity of isolated EVs were assessed by Transmission Electron Microscopy (TEM), size and concentration by Nanoparticle Tracking Analysis (NTA). Western blot and qRT-PCR were used for the analyses of EV specific proteins and micro RNAs, respectively.
Results: The optimized protocol for EV isolation included low speed centrifugation of urine (2.000 x g, RT) for cells removal and storage at -80°C until further analyses. Upon thawing at room temperature, the addition of EDTA inhibited cryoprecipitate formation and uromodulin polymerization. Concentrated PBS was used to neutralize the pH. Sample filtration through 0.22 µm pores enabled removal of larger particles and centrifugal 100kDa membrane units (Amicon®, Milipore) were used to concentrate the sample for particle separation on size-exclusion chromatography (SEC; qEVoriginal, Izon Q). SEC fractions devoid of proteins (as measured at A280) were pooled and concentrated to small volume (70-80 µl). TEM micrographs exhibited high sample purity and typical cup-shaped morphology of isolated EVs. According to NTA, the average mean size of EVs was 129,9 nm and concentration was in the range of 1x109 particles per ml of starting urine. We further confirmed the identity of isolates as EVs by Western blot, as they tested positive for marker proteins Hsc70, flotillin, tubulin, GADPH and CD63. qRT-PCR confirmed the presence of miRNAs in EVs, with CT of 20 for miR let-7i.
Conclusion: We successfully isolated pure population of urinary EVs. The established protocol will be used to assess the potential of EVs and their cargo as non-invasive urinary biomarkers of allograft injury in kidney allograft recipients.
There are no comments yet...