High-mobility group box 1 protein antagonizes the immunosuppressive capacity and therapeutic effect of mesenchymal stem cells in acute kidney injury
Shuo Wang1,3, Songjie Cai2, Weitao Zhang1, Xigao Liu3, Yan Li3, Chao Zhang1, Yigang Zeng1, Ming Xu1, Ruiming Rong1, Tianshu Yang4, Benkang Shi3, Anil Chandraker2, Tongyu Zhu1, Cheng Yang1.
1Department of Urology, Fudan University, Shanghai, People's Republic of China; 2Transplantation Research Center, Renal Division, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, United States; 3Department of Urology, Qilu Hospital of Shandong University, Jinan, People's Republic of China; 4Shanghai Tenth People’s hospital, Shanghai, People's Republic of China
Purpose: Kidney ischemia reperfusion injury (IRI) is a common cause of acute kidney injury and an unavoidable consequence of kidney transplantation and still lacks specific therapeutics. In recent years, mesenchymal stem cell (MSC) emerges as a promising cell-based therapy. The reparative effects of MSC largely dependent on its unique immunosuppressive property, which is primed and regulated by the microenvironments.
Methods: We employed mice kidney IRI model and MSC cell line to monitor the IRI related checkpoints. siRNAs were utilized to knock down the potential key factors for function. Statistical analysis was performed by using one-way ANOVA with Tukey’s post hoc procedure by SPSS.
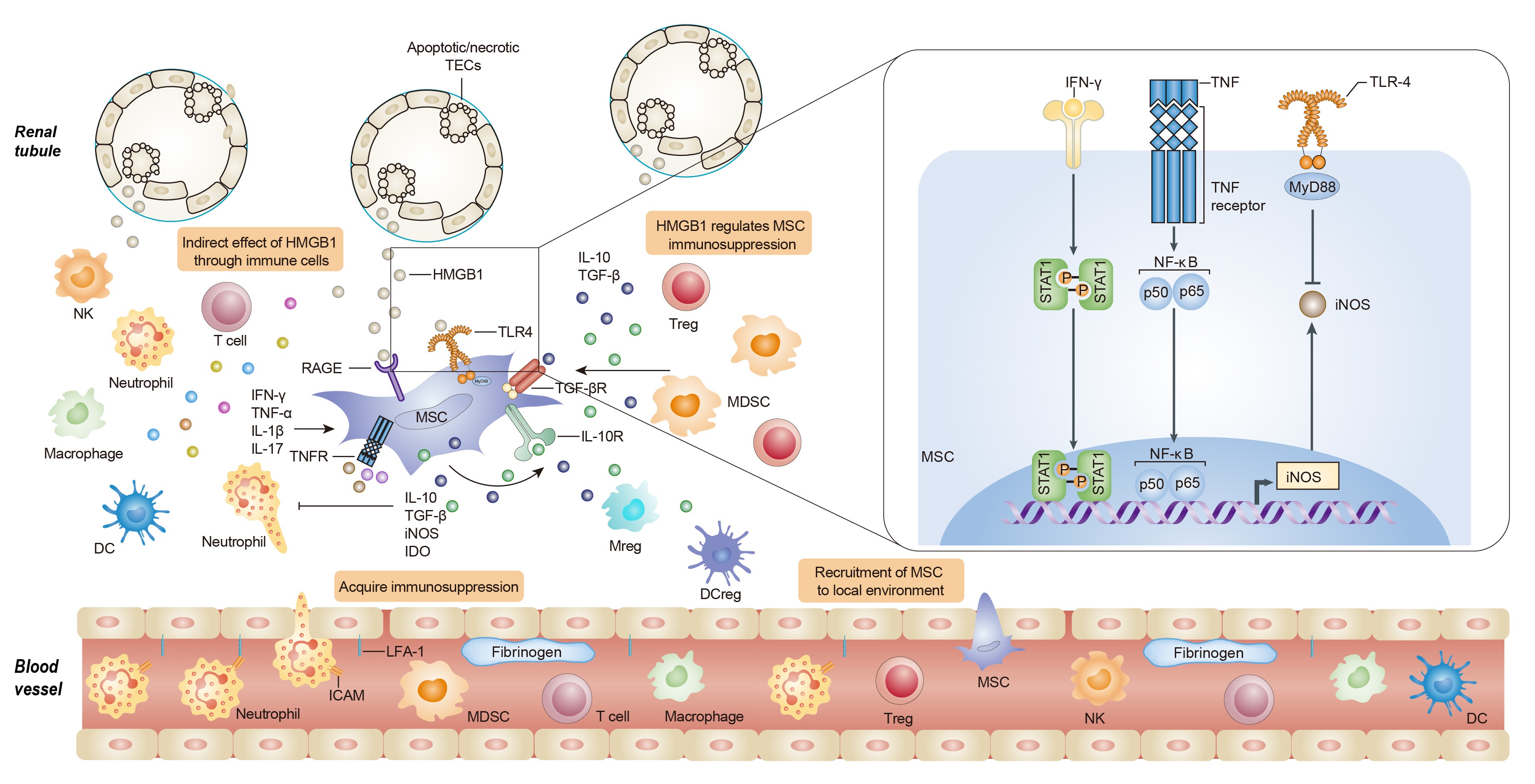
Results: The expression of high-mobility group box 1 protein (HMGB1) is increased not only in the acute phase of IRI, but also during the recovery stage, and the HMGB1 upregulation is correlated with the injury severity. We found that HMGB1 could diminish the immunosuppressive capacity of MSC in the presence of pro-inflammatory cytokines in vitro and in vivo. Toll like receptor 4 (TLR4)-mediated inducible nitric oxide synthase (iNOS) inhibition might contribute to the effect of HMGB1 on MSCs. Importantly, we further demonstrated that HMGB1 blocking strategies would augment the therapeutic efficacy of MSCs on renal IRI.
Conclusions: These findings demonstrate that HMGB1 plays a crucial role in shaping the immunoregulatory property of MSCs within the microenvironments, providing novel insights into the crosstalk between MSCs and microenvironment components, suggesting HMGB1 signals as a promising target to improve MSC-based therapy.
There are no comments yet...