
Assessment of ABO-histocompatibility: Modernizing ABO antibody detection tools for use in transplantation
Anne Halpin1,2,3,4, Bruce Motyka2,3,4,5, Jean Pearcey2,3,4,5, Tess Ellis2,3,4,5, Esme Dijke2,3,7, Todd L. Lowary3,4,6, Chris W. Cairo4,6, Morgan Sosniuk8, Stephanie Maier 3,4,5, Simon Urschel2,3,5, Lori J. West1,2,3.
1Pediatrics and Laboratory Medicine and Pathology, University of Alberta, Edmonton, AB, Canada; 2Alberta Transplant Institute, University of Alberta, Edmonton, AB, Canada; 3Canadian Donation and Transplantation Research Program, University of Alberta, Edmonton, AB, Canada; 4GlycoNet, University of Alberta, Edmonton, AB, Canada; 5Pediatrics, University of Alberta, Edmonton, AB, Canada; 6Chemistry , University of Alberta, Edmonton, AB, Canada; 7Laboratory Medicine and Pathology, University of Alberta, Edmonton, AB, Canada; 8Medical Microbiology, University of Alberta, Edmonton, AB, Canada
Introduction: ABO-incompatible (ABOi) transplantation expands the donor pool for infants awaiting heart transplantation; this is possible as naturally occurring ABO antibodies (Abs) are not present at birth but develop by age 6-12 months. ABOi kidney transplantation can also be undertaken after Ab removal strategies, however unpredictability of risk assessment remains. The current ABO-Ab detection method using red cell agglutination is limited by lack of ABO-subtype specificity, imprecise ABO-Ab isotype differentiation, and poor reproducibility. We previously developed an ABO-glycan microarray to address these limitations. The field of histocompatibility has greatly advanced the accurate characterization of HLA Abs using bead-based tools; our aim here was to create a similar solid phase bead assay for ABO-Ab analysis.
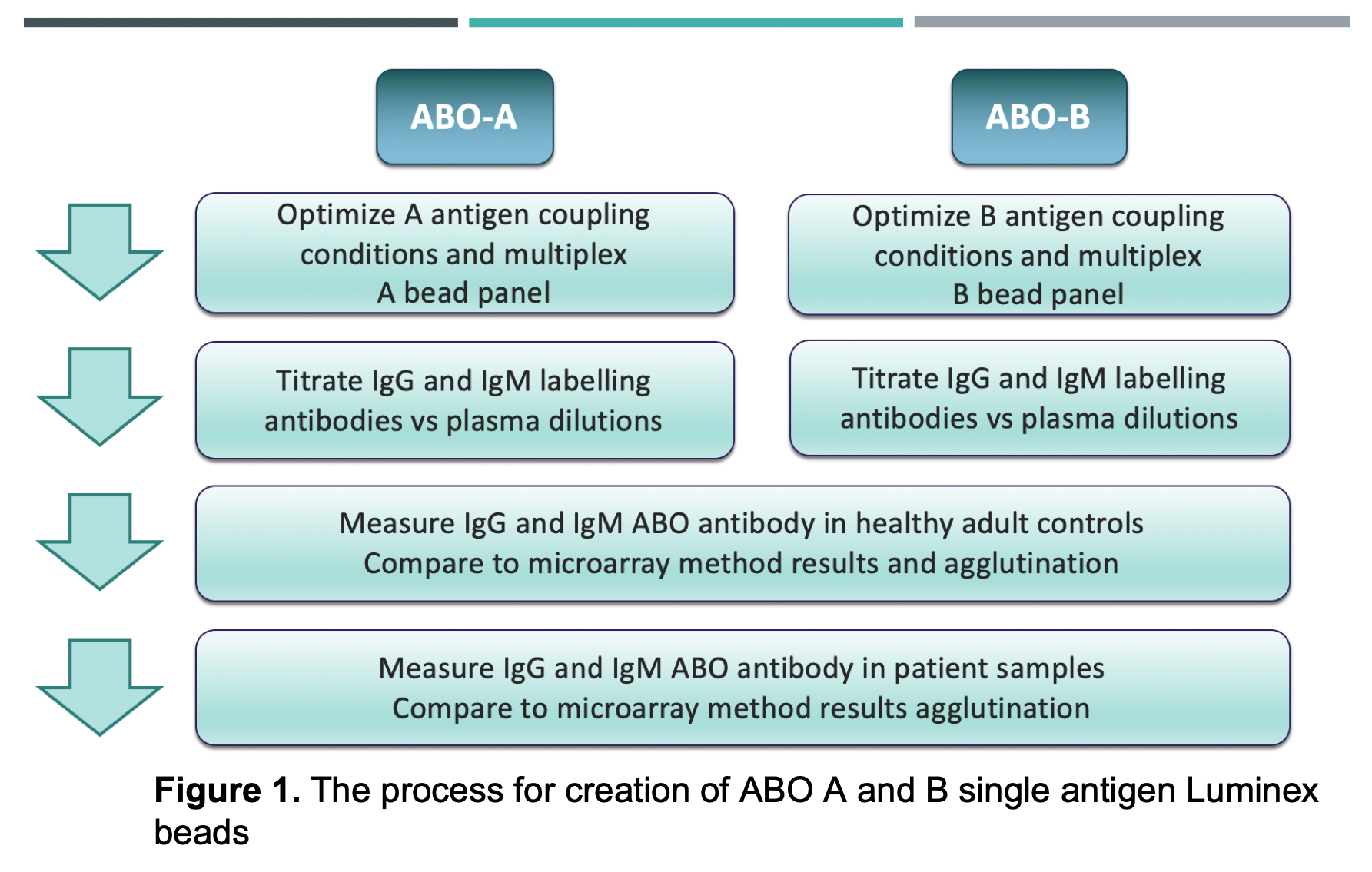
Materials and Methods: ABO A- and B-subtype antigens (I,II,III,IV,V,VI) were coupled to Luminex beads and quantified using monoclonal ABO-Abs. Bovine serum albumin was coupled as a negative control bead. IgG and IgM isotypes with specificities for ABO A- and B-subtypes were measured and compared in healthy adults (n=28) and pediatric patients (n=16) by mean fluorescence intensity (MFI). Samples were also tested on our glycan array and by red cell agglutination. (Overview of methods in Figure 1).
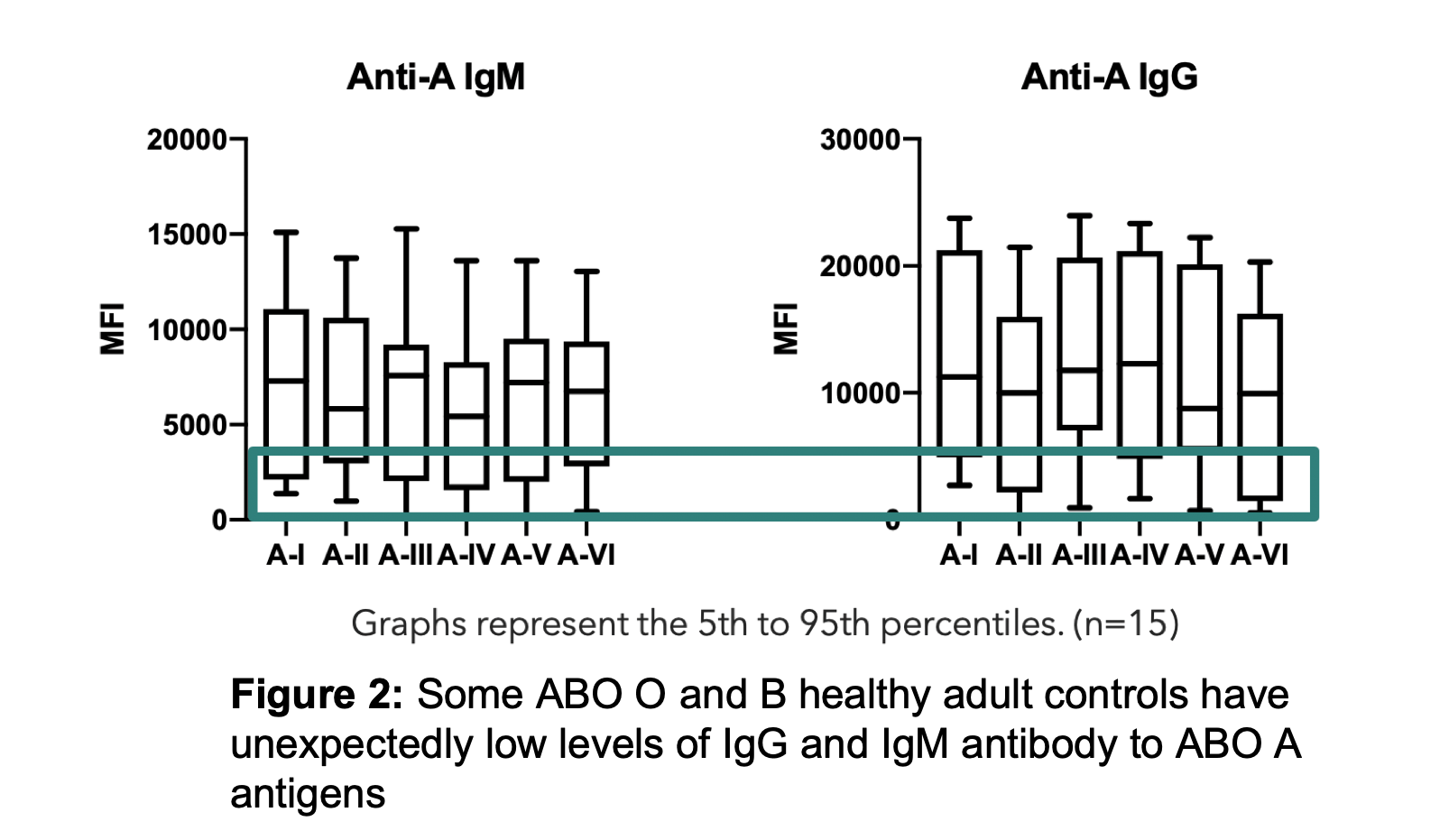
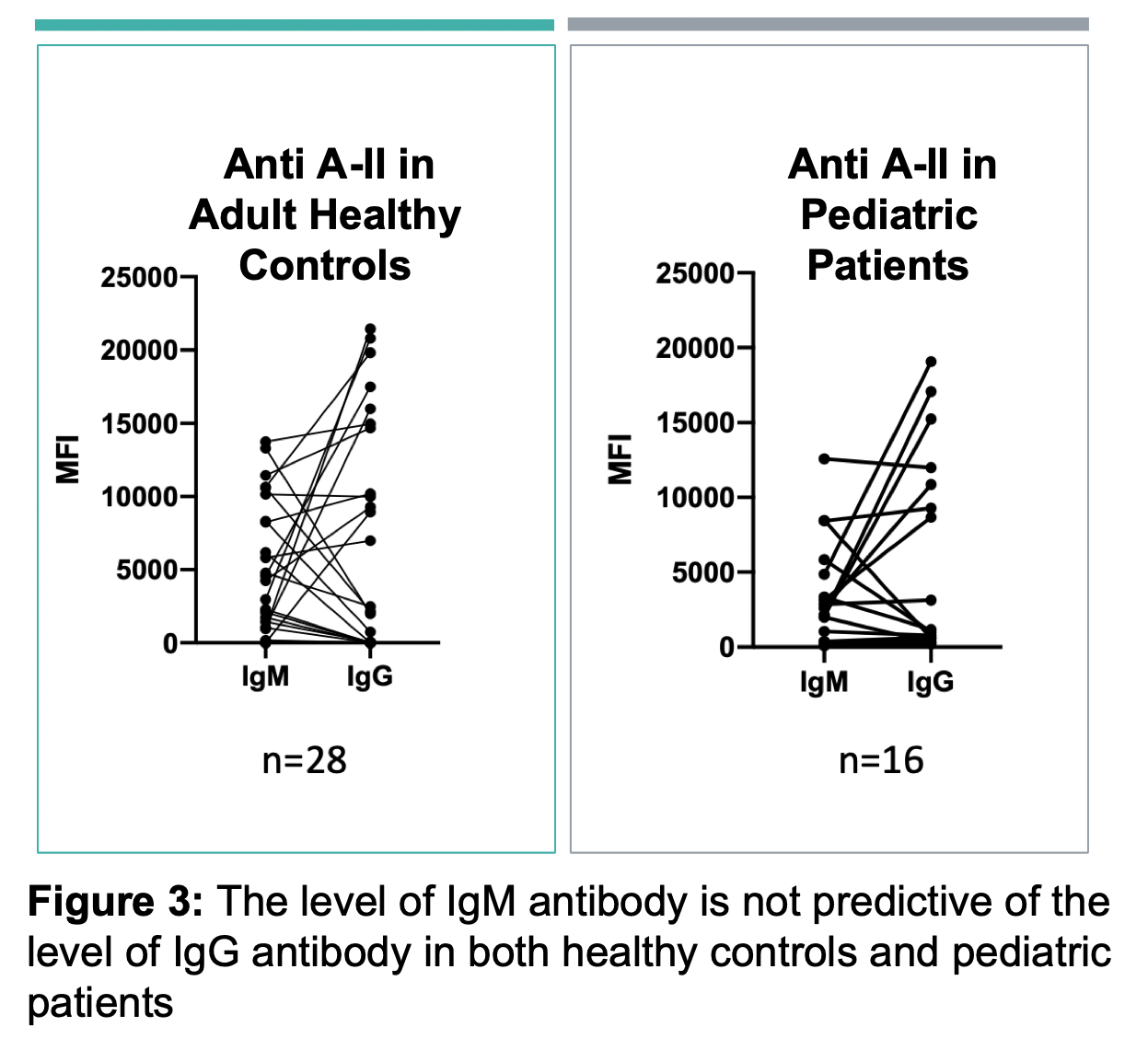
Results: ABO-A and -B subtype-specific Abs were detected with high variability in MFI values amongst subjects. IgG and IgM anti-A and/or anti-B Abs were detectable in non-AB subjects, although MFIs were low in some as shown for anti-A in Figure 2. Anti-A and -B IgM MFIs were similar across individuals of blood group O, compared to B and A subjects (respectively). However, IgG MFIs were higher in O individuals than in A or B subjects. Importantly, the quantity of IgM antibodies did not predict IgG (Figure 3). IgM Ab alone did not predict RBC agglutination titre, as some subjects had mostly IgG ABO Abs not effectively detected by agglutination.
Discussion: Initial results of our Luminex ABO-Ab assay are promising for eventual clinical laboratory development. Histocompatibility laboratories are well-positioned to support this testing as equipment, expertise, and patient samples are already in use. The specificity of this assay will allow precise assessment of ABO-Abs to antigen subtypes, known to be expressed differently in vascular endothelium than red cells. The ability to measure IgM and IgG ABO-Abs makes it possible to evaluate the role of each in potential allograft damage; isotype discrimination may be particularly relevant after plasmapheresis, which more efficiently removes IgM than IgG.
Conclusion: Accurate immune risk assessment in ABOi transplantation relies on well-defined characterization of ABO Abs; improved ABO-Ab detection tools are required. This bead-based assay has the potential to serve as a powerful new tool for precise assessment of risk and for managing care of ABOi transplant patients.
There are no comments yet...