Acquisition and kinetics of EBV infection amongst pediatric liver transplant recipients in Japan
Masaki Yamada1, Akinari Fukuda1, Miyuki Ogura1, Seiichi Shimizu1, Hajime Uchida1, Masahiro Takeda1, Yusuke Yanagi1, Yuriko Ishikawa1, Sakamoto Seisuke1, Mureo Kasahara1, Ken-ichi Imadome1.
1National Center for Child Health and Development, Tokyo, Japan
Purpose: Epstein-Barr virus (EBV) is a ubiquitous virus that infects more than 90% of the adult population worldwide. EBV infection after pediatric liver transplantation (LT) accounts for significant morbidity and mortality because EBV can immortalize the infected cells, resulting in EBV-associated post-transplant lymphoproliferative disorders (PTLD). The polymerase chain reaction (PCR) for EBV has been implemented to monitor the acquisition and the activity of EBV as well as the proliferation of EBV-infected cells to prevent the development of PTLD. However, optimal timing and frequency of EBV monitoring protocol remain uncertain. In Japan, the majority of pediatric LT is performed from living adult donors, and the detailed epidemiologic data of EBV infections and their outcomes in this cohort is lacking. Hence, the purpose of this study is to describe the epidemiology of EBV infection after living donor dominant pediatric LT cohort and reveal the kinetics of EBV loads by using a longitudinal data set of EBV PCR monitoring, and further to establish the optimal monitoring protocol.
Methods: We retrospectively reviewed clinical and laboratory data from 520 patients who underwent isolated LT between 2006/1/1 and 2018/12/31 at the tertiary children’s hospital in Japan. Further analysis was performed amongst 407 children who were <18 years of age at the time of LT and had at least five EBV load measurements throughout the course. The frequency of EBV monitoring was determined per clinical protocol. EBV DNA loads were reported in both copy/μgDNA in blood cells and copy/mL in plasma, as previously described. The reduction of immunosuppressants is usually attempted when the EBV loads are above either 1000 copy/μgDNA or copy/mL, respectively. P<0.05 is considered statistically significant.
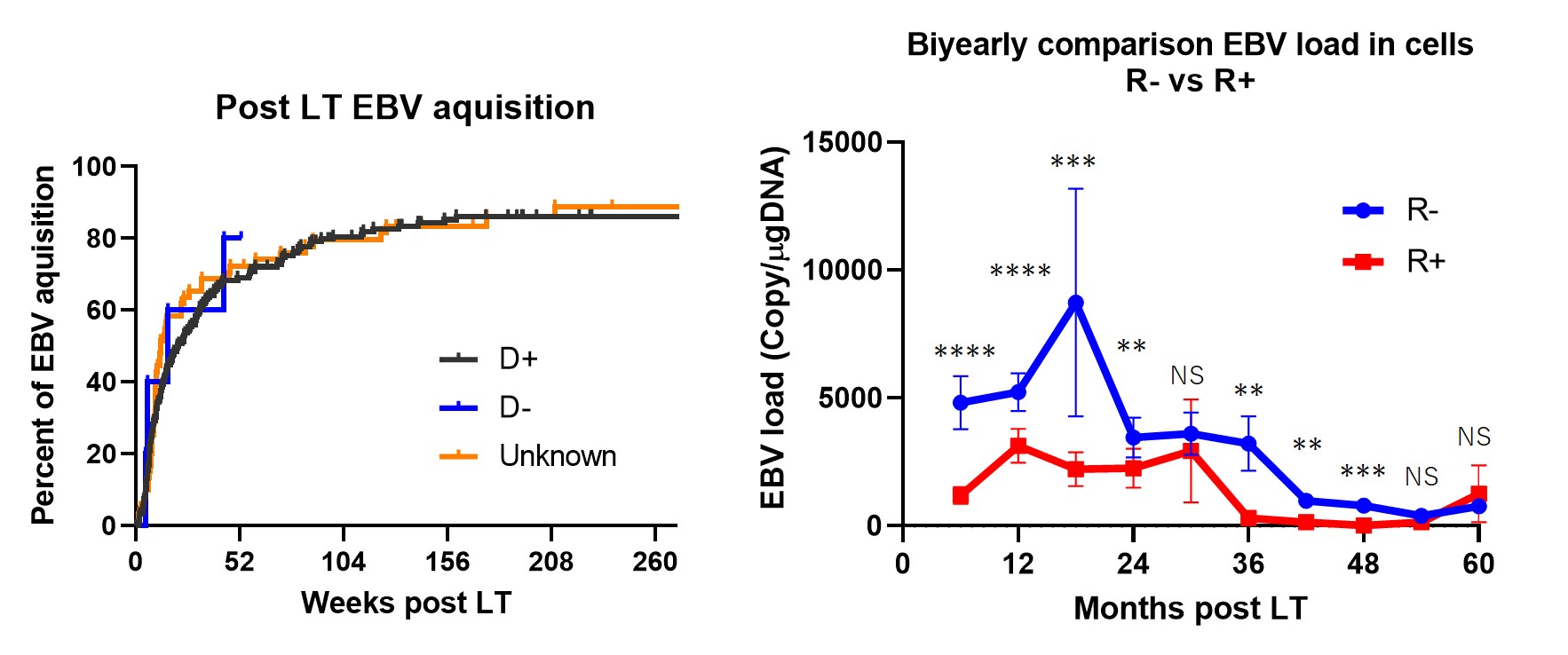
Results: A total of > 5000 data points were analyzed. 94% of the study subjects underwent living donor LT. (1) 80% of EBV naïve LT recipients (R-) acquired primary EBV infection within two years post LT. (2) EBV loads of R- were higher than that of R+ in blood cells, especially in the first two-years post LT. In contrast, EBV loads after four-years post LT were not different. (3) There were no PTLD patients in our cohort.
Conclusions: Most of our cohort underwent living donor LT. Although EBV acquisition/reactivation frequently occurred early after LT in both R- and R+ in the early post LT period, the EBV loads were higher in R- groups in the first two years, and were similar after four-years post LT. Our results indicate that four years of proactive EBV monitoring may be sufficient to intervene in active EBV infection after pediatric living donor LT.
[1] Allen, U. D., Preiksaitis, J. K. & Practice, ASTIDCP. Post-transplant Lymphoproliferative Disorders, EBV infection and Disease in Solid Organ Transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant, e13652, doi:10.1111/ctr.13652 (2019).
[2] Yamada, M. et al. Epidemiology and outcome of chronic high Epstein-Barr viral load carriage in pediatric kidney transplant recipients. Pediatr Transplant, doi:10.1111/petr.13147 (2018).
[3] Imadome, K. et al. Effective control of Epstein-Barr virus infection following pediatric liver transplantation by monitoring of viral DNA load and lymphocyte surface markers. Pediatr Transplant 16, 748-757, doi:10.1111/j.1399-3046.2012.01750.x (2012).
There are no comments yet...