A proof of concept study for [18F]-sodium fluoride imaging of nephrocalcinosis in donor kidneys and explanted renal allografts
Stan Benjamens1,2, Ines F. Antunes2, Jan-Luuk Hillebrands3, Stefan P. Berger4, Martin H. de Borst4, Riemer H.J.A. Slart2, Robert A. Pol1.
1Division of Transplantation Surgery, Department of Surgery, University Medical Center Groningen, Groningen, Netherlands; 2Medical Imaging Center, University Medical Center Groningen, Groningen, Netherlands; 3Pathology, University Medical Center Groningen, Groningen, Netherlands; 4Internal Medicine, University Medical Center Groningen, Groningen, Netherlands
Dutch Kidney Transplant Study Group.
Introduction: Nephrocalcinosis is an important factor in the decline of graft function after kidney transplantation and is associated with both inferior graft function and poor graft survival. In invasive kidney allograft biopsies, microcalcifications are demonstrated in 12% - 43% of biopsies at one-year up to 79% at 10-years after kidney transplantation. As first step towards a potential non-invasive imaging method for identification of kidney microcalcifications, we performed a proof of concept study for ex-vivo [18F]-sodium fluoride ([18F]-NaF) imaging in donor kidneys and explanted renal allografts.
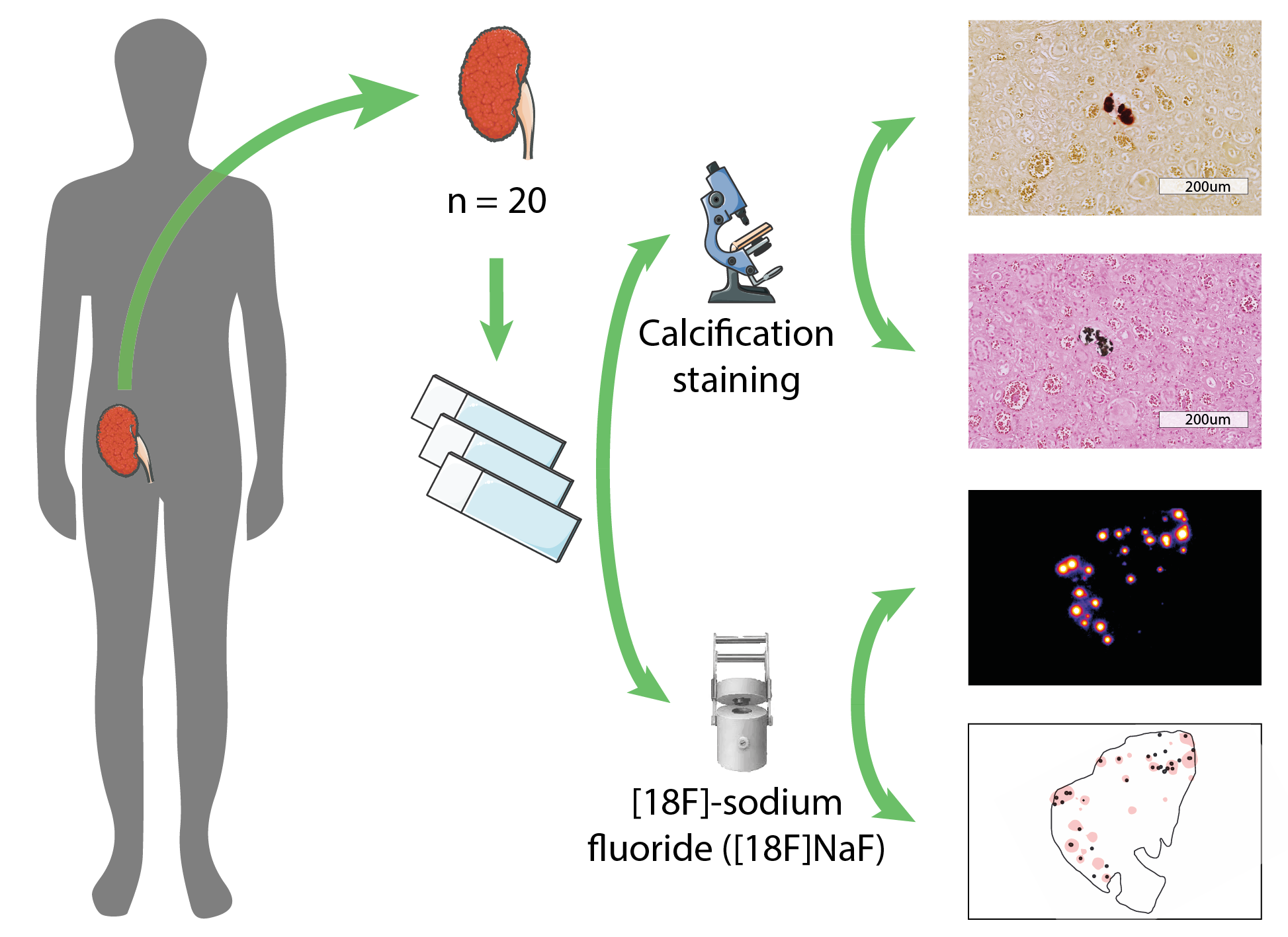
Materials and Methods: Human kidney samples were obtained after deceased organ donation (n = 7) and after transplantectomy (n = 13). Three µm paraffin-embedded sections were used for histological evaluation of calcification (Alizarin Red and Von Kossa staining) and autoradiography imaging after a 30 minutes incubation with the radiopharmaceutical [18F]-NaF. Semi-automated detection of calcifications in tissue sections was performed and areas of [18F]-NaF activity were identified, resulting in overlay images of histology and tracer activity (Figure 1). The calcifications were classified according to their localization in the glomerular or tubulointerstitial area. Correlations between histology and autoradiography findings were presented as Spearman’s rho.
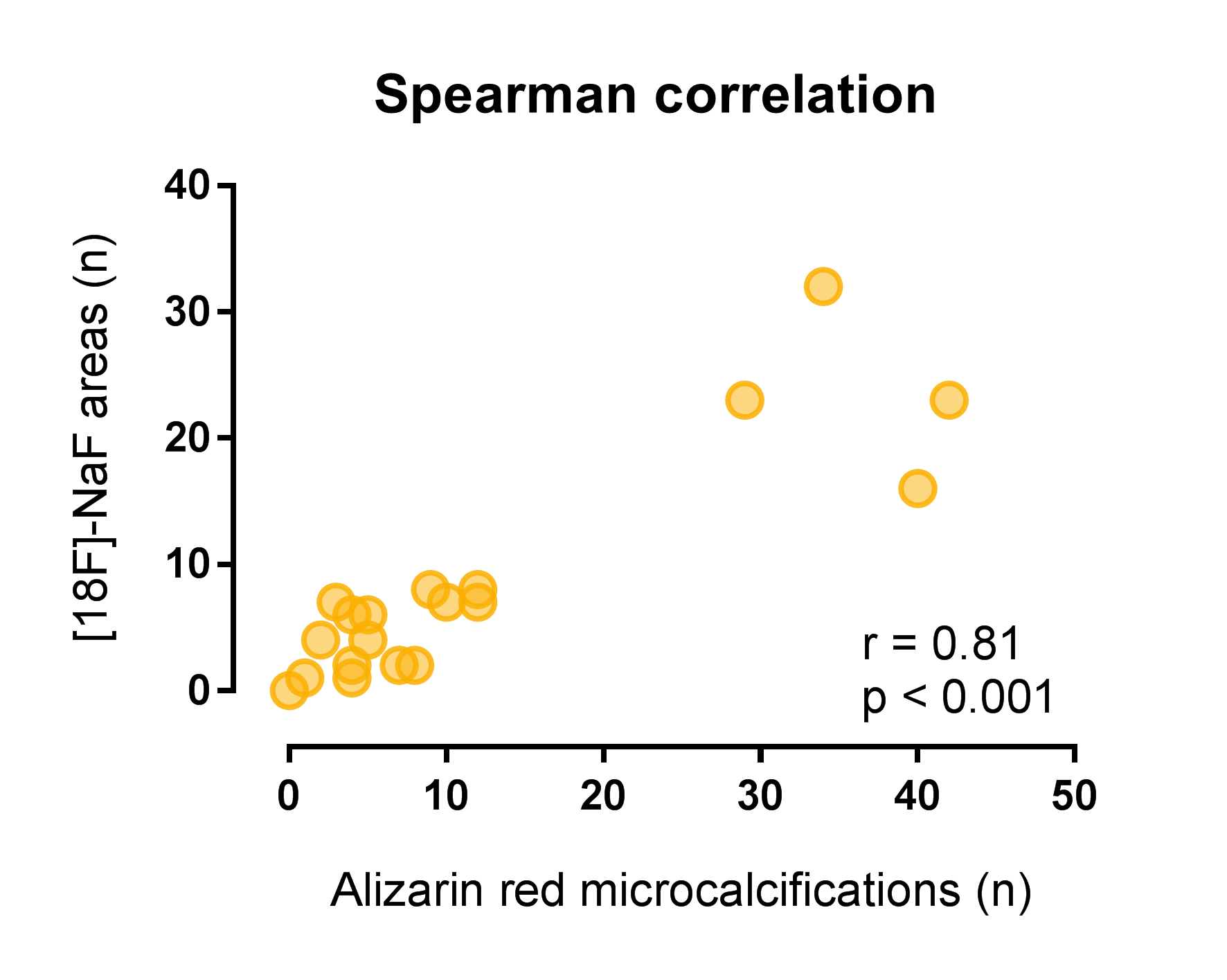
Results: Transplantectomy samples were obtained at a median of 33 (IQR 9 – 66) months after transplantation, deceased organ donor samples were obtained from discarded kidneys. Microcalcifications were identified in 19 out 20 Alizarin Red stained samples, predominantly in cortical tissue. Tubulointerstitial microcalcifications were seen in 19 out 20 samples, with a median of 5.5 (IQR 2 – 10.3) per samples, and glomerular microcalcifications were seen in 7 out 20 samples, with a median of 1 (IQR 1 – 3) per sample. With autoradiography, [18F]-NaF activity was found in 19 out 20 samples, with an area surface of tracer activity of ≤5% in 13 and >5% in 6 out of 20 samples. Alizarin Red staining of microcalcifications demonstrated good correlation with [18F]-NaF activity (Spearman’s rho of 0.81, P<0.001) (Figure 2), whereas Von Kossa staining demonstrated weak correlation with [18F]-NaF activity (0.59, p=0.006).
Conclusion: In this proof of concept study, we demonstrated that ex-vivo [18F]-NaF binding in human kidney parenchym samples has good correlation with histology-proven microcalcifications. This is the first step towards a non-invasive imaging method to identify microcalcifications in active nephrocalcinosis. The use of [18F]-NaF may lead to better understanding of the role of microcalcifications in the decline of graft function after transplantation.
There are no comments yet...